4.1 Selection of Materials
4.1.1 By selecting a metal least likely to corrode in the design phase the potential for costly corrosion repairs is reduced. This can be achieved in a number of ways –
a. Substitution of metals with other less-corrosive materials such as stainless steel, aluminium or even plastics. For example the render and ceilings and linings manual/policy call for the use of PVC trims in lieu of steel to avoid the potential for rust to cause damage where a suitable alternative is available.
b. Separation of metals to prevent galvanic and fretting corrosion. This can simply be the use of plastic spacers or washers between reactive metals.
4.1.2 For example if these were stainless screws on colourbond the metals are dissimilar. In this case the rubber washers isolate the two materials isolating the two materials and preventing corrosion. This is a simple explanation however the principle is the same on all materials.
4.2 Protective Coatings
4.2.1 Passive
a. Passive barrier protection works by coating the steel with a protective coating system that forms a tight barrier to oxygen, water and salts (ions).
b. The lower the permeability of the coating system to water, the better the protection.
c. Two-pack epoxy coatings and chlorinated rubbers applied at sufficiently high film builds offer the most successful corrosion protection through passive barrier protection.
4.2.2 Active
a. The most common form of protection for structural steel (Internal) paint systems that are typically rich in zinc.
b. Paints are not impervious to air so will eventually promote corrosion of the base metal where not maintained or embedded.
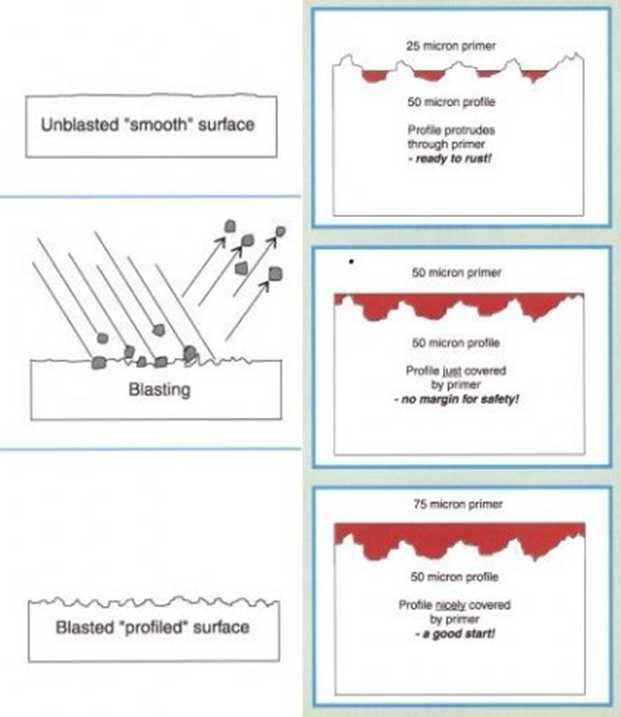
c. Preparation of the surface prior to application of any paint system is critical and is achieve by sand or shot blasting to set a Class depending on the level of protection required.
d. Immediately following surface preparation a shop primer is applied. This primer may be required to be removed or form the base of subsequent coats of a more substantial paint system.
4.2.3 The thickness of the applied paint will directly affect the life of the system and needs to be compatible with the class of surface preparation as demonstrated below.
4.2.4 Active corrosion protection occurs when a primer containing a reactive chemical compound is applied directly to the steel.
4.2.5 The reactive compound disrupts the normal formation of anodes on the surface of the steel in some way. For example, inorganic zinc inhibitive pigments, such as zinc phosphate, offer active anti-corrosive protection to the steel substrate. The zinc ions act as cathodic inhibitors.
4.2.6 The atmospheric corrosion rate of zinc is approximately ten times slower than that of black or untreated steel which is why it makes such a good protective layer however it may be applied. The corrosion rate of zinc is:
• rural atmosphere: < 1 µm/year
• urban atmosphere: ≈ 2 µm/year
• industrial atmosphere: 2 ... 10 µm/year
• marine atmosphere: ≈ 2 µm/year
4.3 Preparation
The Australian Standard 1627 Series gives guidance on the selection of the appropriate methods for the preparation and pre-treatment of metal surfaces prior to the application of a protective coating.
4.3.1 Sacrificial Protection (Galvanic Protection)
a. Involves coating the surface of steel with a sacrificial corrosion-resistant metal such as zinc which protects steel. Like paint it provides barrier protection from the elements but importantly it provides galvanic protection whereby the zinc will sacrifice itself to protect steel.
b. The most widely used metal for the protection of steel is zinc. Zinc metal in direct contact with the steel substrate offers protection through the preferential oxidation of zinc metal. Zinc is a great choice in protecting steel, as not only does it corrode in preference to the steel, the RATE of corrosion is generally lower. This rate, however, is accelerated in the presence of ions such as chlorides in coastal locations.
c. Examples of coatings using this principle of sacrificial protection include inorganic zinc silicates, organic zinc-rich primers, metal-sprayed zinc, hot dip galvanising and electroplating.
4.3.2 Sacrificial Protection (Cathodic Protection)
a. Method to reduce corrosion by minimizing the difference in potential between anode and cathode.
b. Cathodic protection is achieved by applying a current to the structure to be protected (such as a pipeline) from some outside source. When enough current is applied, the whole structure will be at one potential; thus, anode and cathode sites will not exist. Cathodic protection is commonly used on many types of structures, such as pipelines, underground storage tanks, locks, and ship hulls.